Details of the Drug
General Information of Drug (ID: DMOY7W3)
| Drug Name |
Artemisinin
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Arteannuin; Artemisine; Artemisinina; Artemisinine; Artemisininum; Artesin; Huanghuahaosu; Qinghaosu; Qinghosu; Quinghaosu; Artemisinin [INN]; Artemisinina [Spanish]; Artemisinine [French]; Artemisininum [Latin]; Qing Hau SU; Qing Hau Sau; Qing Hau Sau [Chinese]; Qinghaosu [Chinese]; Quing Hau Sau; Artemisinin (INN); Coartem, Artemisinin; Artemisia annua L., extract; GNF-PF-5671; GNF-Pf-5341; Octahydro-3,6,9-trimethyl-3,12-epoxy-12H-pyrano(4,3-j)-1,2-benzodioxepin-10(3H)-one; (+)-Arteannuin; (+)-Artemisinin; (3R,5aS,6R,8aS,9R,12S,12aR)-3,6,9-trimethyloctahydro-3,12-epoxypyrano[4,3-j][1,2]benzodioxepin-10(3H)-one; (3R,5aS,6R,8aS,9R,12S,12aR)-Octahydro-3,6,9-trimethyl-3,12-epoxy-12H-pyrano(4,3-j)-1,2-benzodioxepin-10(3H)-one; (5aS,6R,8aS,9R,12S,12aR)-3,6,9-trimethyloctahydro-3,12-epoxy[1,2]dioxepino[4,3-i]isochromen-10(3H)-one; 1,5,9-trimethyl-(1R,4S,5R,9R,12S,13R)-11,14,15,16-tetraoxatetracyclo[10.3.1.04,13.08,13]hexadecan-10-one; 3,6,9-trimethyloctahydro-3,12-epoxy[1,2]dioxepino[4,3-i]isochromen-10(3H)-one
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
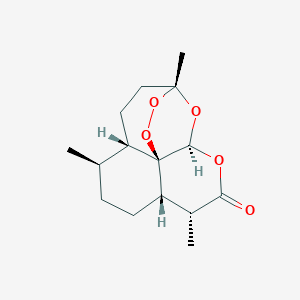 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 282.33 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Malaria | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 1F40-1F45 | |||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||
References
